How CDC Determines the Level for COVID-19 Travel Health Notices
April 25, 2022
CDC uses Travel Health Notices (THNs) to alert travelers and other audiences to health threats around the world and advise on how to protect themselves.
On April 18, 2022, CDC updated its COVID-19 THN system. Level 4 will no longer be based on COVID-19 incidence or case count alone. It will be reserved for special circumstances, such as rapidly escalating case trajectory or extremely high case counts, emergence of a new variant of concern, and healthcare infrastructure collapse. Levels 3, 2, and 1 will still be primarily determined by 28-day incidence or case counts as outlined below.
COVID-19 Travel Recommendations can be found in two places:
- Interactive world map showing COVID-19 travel recommendations by destination
- CDC’s COVID-19 Travel Health Notice webpage
The 4-level system categorizes international destinations into the following levels:
Level 4: Special Circumstances / Do Not Travel
- Do not travel to this destination.
- If you must travel, make sure you are up to date with your COVID-19 vaccines before your trip.
Level 3: High Level Of COVID-19
- Make sure you are up to date with your COVID-19 vaccines before traveling to this destination.
- If you are not up to date with your vaccines, avoid travel to this destination.
- If you have a weakened immune system or are more likely to get very sick from COVID-19, even if you are up to date with your COVID-19 vaccines, talk with your clinician about your risk and consider delaying travel to this destination.
Level 2: Moderate Level Of COVID-19
- Make sure you are up to date with your COVID-19 vaccines before traveling to this destination.
- If you have a weakened immune system or are more likely to get very sick from COVID-19, even if you are up to date with your COVID-19 vaccines, talk to your clinician about what additional precautions may be needed before, during, and after travel to this destination.
Level 1: Low Level of COVID-19
- Make sure you are up to date with your COVID-19 vaccines before traveling to this destination.
Level Unknown: Unknown Level of COVID-19
- Make sure you are up to date with your COVID-19 vaccines before traveling to this destination.
- If you are not up to date with your vaccines, avoid travel to this destination.
- If you have a weakened immune system or are more likely to get very sick from COVID-19, even if you are up to date with your COVID-19 vaccines, talk with your clinician about your risk, and consider delaying travel to this destination.
Travel Health Notice Thresholds
CDC reviews case data reported to the World Health Organizationexternal icon to determine a destination’s COVID-19 THN level.
Level 4 Travel Health Notices
Level 4 will be reserved for special circumstances, such as rapidly escalating case trajectory or extremely high case counts, emergence of a new variant of concern, and healthcare infrastructure collapse. Other factors that may be considered include information such as vaccination rate and hospitalization rate. CDC works with country authorities through CDC country or regional offices to gather additional data as appropriate.
Level 1-3 Travel Health Notices are determined as follows:
Primary criteria for destinations with populations over 100,000
- Incidence rate (cumulative new cases over the past 28 days per 100,000 population)
- New case trajectory (Have daily new cases increased, decreased, or remained stable over the past 28 days?)
Primary criteria for destinations with populations of 100,000 or less
- COVID-19 case counts* (cumulative new cases over past 28 days)
- New case trajectory (Have daily new cases increased, decreased, or remained stable over the past 28 days?)
*CDC does not count identified imported cases (i.e., cases in travelers who were exposed in another country) against a destination’s total.
Secondary Criteria for Determining Travel Health Notice Levels
Reported case counts and incidence rates depend on testing capacity. CDC assesses testing capacity using two secondary criteria metrics: population testing rate and test-to-case ratio. The population testing rate is the number of tests conducted per 100,000 people over 28 days. The test-to-case ratio is the number of tests conducted for each case reported during the same 28-day period. Testing data are obtained from multiple sources, including Our World in Dataexternal icon, Foundation for Innovative Diagnosticsexternal icon, and country ministries of health.
Travel Health Notice levels 1 through 3 for destinations with a population more than 100,000 people. Levels are based on combined 1) incidence rate (primary criteria) and 2) testing data (secondary criteria)
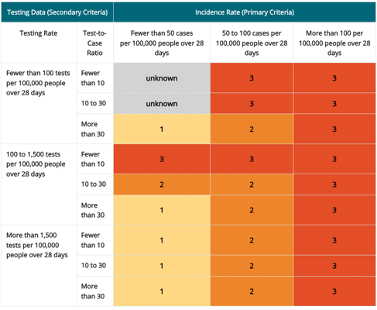
*Incidence rate is the primary criteria for destinations with a population more than 100,000 people. Testing data are the secondary criteria and that data includes both the testing rate (column 1) and test-to-case ratio (column 2). The resulting THN levels are shown in rows 3–11 of columns 3–5.
Travel Health Notice levels 1 through 3 for destinations with a population of 100,000 people or fewer. Levels are based on combined 1) case count (primary criteria) and 2) testing data (secondary criteria) *
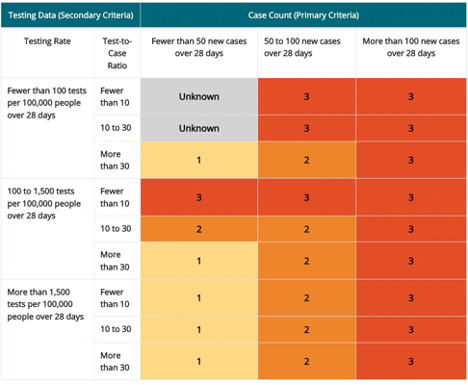
*Case count is the primary criteria for destinations with a population fewer than or equal to 100,000 people. Testing data are the secondary criteria and that data includes both the testing rate (column 1) and test-to-case ratio (column 2). The resulting THN levels are shown in rows 3–11 of columns 3–5.
Population testing rates of more than 1,500 tests per 100,000 people over 28 days are considered sufficient to provide an accurate representation of COVID-19 in the destination. Rates less than or equal to 1,500 tests per 100,000 people over 28 days may signify concerns that testing is insufficient and may not provide an accurate representation of the incidence rate in the destination. The cutoffs for evaluating population testing ratesexternal icon have been adapted from the WHO guidelines.
The WHO determined a test-to-case ratio greater than or equal to 10 as the minimum indicator of sufficient surveillance capacity. A test-to-case ratio of less than 10 tests per case might indicate restrictive testing, or that only symptomatic people are being tested and undercounting the incidence rate (primary criteria). The preferred level is a test-to-case ratio of more than 30. The cutoffs for evaluating test-to-case ratios pdf icon[PDF – 18 pages]external icon have been adapted from the WHO guidelines.
When both the population testing rates and test-to-case ratios are high, CDC has confidence in a destination’s reported incidence. If either the population testing rate or test-to-case ratio is low, CDC has less confidence that the reported incidence accurately depicts the COVID-19 situation in the destination. In this situation, CDC adjusts a destination’s THN level as shown in the tables above. Countries with low incidence and testing rates are classified as unknown as well as countries that report data infrequently.
Level Unknown Travel Health Notices are determined as follows:
If a destination has insufficient data to make a THN level determination, its THN level is designated as “unknown”. Insufficient data means that the destination does not provide data or that the provided data are non-representative of the COVID-19 situation in the destination, making an accurate THN level determination difficult. This situation includes destinations with low COVID-19 incidence and low reported COVID-19 testing levels.
Raising a Travel Health Notice
CDC raises a destination’s THN level when the incidence rate (or case count) and testing metrics meet the THN threshold for a higher level and remain at that level for 14 consecutive days. The THN level may be raised before 14 days if there is a large increase in COVID-19 cases reported.
Lowering a Travel Health Notice
CDC lowers a destination’s THN level when the incidence rate (or case count) and testing metrics meet the THN threshold for a lower level and remain at that level for 28 consecutive days. Vaccination coverage rates and case trajectory will be considered when determining if the THN level can be lowered before 28 days.
For more information, visit How CDC Determines the Level for COVID-19 Travel Health Notices | CDC